Quality Management Systems (QMS) Consultants
What is a Quality Management System?
A Quality Management System (QMS) is an assemblage of processes, procedures, and polices for achieving customer requirements and customer satisfaction. These systems, when appropriately applied, can provide an enterprise with increased operational consistency and profitability.
Although originally created for manufacturing endeavors, QMS are also applicable for service industries. There has been a marked increase in the adoption of QMS across a wide sector of industries. In fact, in many industries, it is a requirement to have a viable quality system. Today QMS is practiced in areas such as forestry, food processing, aerospace, medical devices manufacturing, retail, and financial services.
Types of QMS
Because of the requirement for uniformity of application, QMS are built around standards. One of the earliest and most popular standards is ISO 9001. Its current revision is ISO 9001:2015. The ISO 9001 standard is applicable to a wide variety of industries and is being increasingly implemented by businesses.
There are numerous other standards for quality management. Each meet the specific needs of a business sector. Some of these ISO standards include:
- AS 9100D: Quality Management Systems – Requirements for Aviation, Space, and Defense Organizations
- ISO 13485 for Medical Devices
- ISO 14971 for Medical Devices – Application of Risk Management to Medical Devices
- ISO 27001 Information security, cybersecurity and privacy protection — Information security management systems — Requirements
Certification in any of these standards provides a level of assurance among businesses working together in their respective fields. It provides evidence that their products and or services will meet the requirements for their industries and thereby meet customer expectations.
The Benefits of Adopting a Quality System
As previously mentioned, a QMS can provide assurance that a given product or service consistently meets or exceeds customer requirements. In turn this can expand an organizations client base, ensure repeat customers, and increase sales.
It can also, however, can increase the efficiency of an organization by reducing waste, preventing product recalls, and streamlining processes. Furthermore, it can ensure that the organization’s requirements are harmonized with the specific regulations of its industry thereby eliciting trust in its partners and protecting its reputation.
Other benefits of using a QMS include:
- Increased accountability of that encourages participation of employees
- Organizational flexibility to meet changes in the marketplace
- Establishment of goals that encourage growth
- Improved company morale
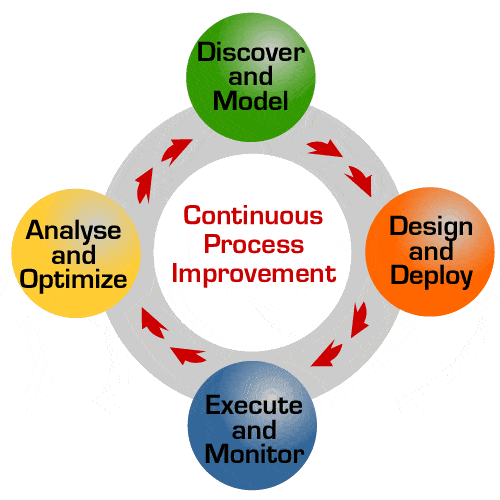
The Dynamics of Quality
An important feature of managing quality is continuous improvement. Continual improvement is, as one might surmise, is an ongoing effort to improve the business processes involved in producing a quality product. These improvements can occur incrementally or be introduced in a large “breakthrough”.
Regardless of how they are implemented, they are instituted through analysis of metrics. These same metrics can then be used to monitor the effectiveness of those changes. In a well-designed program, this feedback is conducted by all stakeholders so that a complete analysis can be provided.
When establishing objectives, every challenge is an opportunity to achieve excellence. Organizations that strive for continuous improvement will perform more efficiently and remain competitive. This improvement can be made more viable and measurable if objectives are clearly researched and defined.
To adequately research causes and effective corrective measures it is important to engage an organization at all levels because front-line workers can often provide a more granular perspective than an organization’s managers and senior leaders. By affording all team members the opportunity to provide feedback, an organization can nurture a culture of continuous improvement that will provide results.
Requirements for Implementing an Effective QMS
Assessment
As with any tool, desired results are a product of implementation. The first requirement for implementing a QMS is to tailor the program to meet the specific needs of the organization.
This process is described in ISO 9001 as context of the organization. It includes risk assessments and risk analysis of the internal and external issues that would affect the governance and implementation of the program. These would include organizational structures, standards adopted by the organization, product, social and cultural issues, market issues and trends, and external stakeholder relationships.
This process also includes capturing the specific requirements for an organization. This would include customer requirements and expectations, applicable regulatory requirements, and desired levels of financial performance.
Creation of the QMS
Once an assessment has been conducted policies, and procedures can be constructed that incorporate a strategy to meet the identified requirements. During this phase, key stakeholders are identified who will assume responsibility for various functions of the system. Inclusion of top management is especially important because by in from the top is essential for a QMS to produce favorable results. It is also important because of the need for effective management review.
Certification and Auditing
Once a QMS has been created, a third party can evaluate the program and its documents to ascertain if the program meets the requirements of the standard, thereby my meriting certification.
Having acquired certification the organization is then required to perform periodic internal audits and external audits to ensure that the program stays on track or can benefit from corrections.
CVG Strategy
Our experience with ISO 9001:2015 , AS9100D, ISO 27001:2013, AAR M-1003 and ISO 13485:2016 adds value to your Quality Management System implementation. Our services include training, document creation and guidance to your management team and all employees.
CVG Strategy quality experts focus on a process approach towards improvement in all our work. Understanding quality systems is a fundamental aspect of our work as consultants, helping our customers make their business run more efficiently and to improve customer satisfaction.
For over a decade, we have helped numerous organizations within various business sectors institute and maintain their quality management systems. We have assisted businesses in the medical device industry, defense, aerospace, and with information security management.
CVG Strategy Quality Management experts are Exemplar Global certified as Lead Auditors for ISO 9001:2015, ISO 13485, FDA QSR, and ISO 27001. We also provide essential training in an engaging format.
How Can We Help?
Latest News
Nicaragua Export Restrictions Increased by U.S.
Nicaragua export restrictions have been increased by both the Directorate of Defense Trade Controls (DDTC) and the Bureau of Industry and Security (BIS) as of


