Risks and Opportunities are Everywhere
Businesses evaluate risks and opportunities every time they make a decision. It is all about weighing the probability of a positive outcome versus the impact or cost of a negative outcome from an action taken.
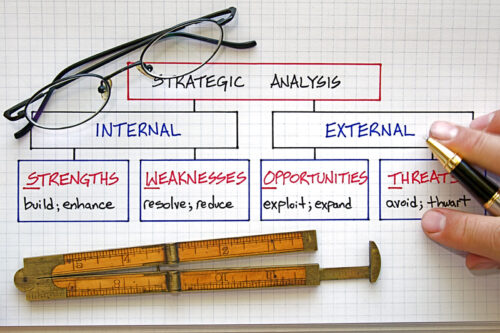
Strengths, Weaknesses, Opportunities, and Threats (SWOT)
For most organizations ISO 9001:2015 provides a Quality Management System (QMS) that can address the risks and opportunities. This can be accomplished in a number of ways including performing a Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis. A SWOT analysis enables businesses to identify strengths and weaknesses. It can also provide information that allows informed decision making in adapting business models.
Risks and Opportunities play a role in continual improvement and should be implemented into your regular improvement processes. Prior to approving and implementing a quality plan, corrective action, non-conformance, design and development plan are all areas that require “risk based thinking”. These quality processes (and more) are required and used by most companies who apply a formal and organized risks and opportunities procedure.
By identifying the risks and opportunities that are applicable to an organization, appropriate actions can be taken to take to limit the negative impacts of potential problems. These actions can also allow an organization to capitalize on opportunities that can lead to new sales markets or product lines. Properly acting on risks and opportunities can realize great profit for your company and help resolve a fundamental requirement in ISO 9001:2015 for “risk based thinking”.
Risk Management in Medical Device Manufacture
For manufacturers of medical equipment, risk management is required to ensure products that can safely perform their designed tasks. For medical device manufacturing ISO 14971:2019 establishes general requirements for risk management.
The standard includes specification for risk analysis, risk evaluation, and risk control execution. It establishes the requirements for conducting evaluations of residual risk. It also provides process criteria for risk management review. Finally, the standard establishes requirements for gathering data of equipment during production and post production phases of the device life cycle.
The standard contains three annexes that contain rationale for the requirements, provide details for risk management processes, and define basic risk concepts. It also includes a Guidance Document, ISO/TR 24971:2020, that contains eight informative annexes providing detail on a variety of issues including the identification of risk, the roles and relationships between policies, risk acceptability, risk control, and risk evaluation and special guidance for vitro diagnostic medical devices.
Aerospace Industry Applications
Aerospace is an industry that must effectively address risk management. AS9100 is the applicable standard for this industry. This standard uses a High-Level System (HLS). This HLS allows ease of use between standards and helps companies implement complex multi-standard quality management systems.
CVG Strategy’s AS9100 Consulting Experts have prepared several multi-standard quality management systems for its customers (AS9100D & ISO 9001:2015 & Aerospace Customer Standard, AS9100D & AAR M-1003 [Rail Roads]).
AS9100D addresses key concerns of the aviation, space, and defense industries. These include:
- Increased emphasis on Product Safety.
- Increased emphasis on the requirement for risk assessment in operational processes.
- Consideration of human factors in the work environment e.g. (distraction, fatigue, lack of resources, lack of knowledge).
- Improvement in stakeholder requirement assessment through configuration management.
- A reinforcement for individual awareness of product and service quality and safety. This also pertains to ethical behavior in the performance of these tasks.
- Measures to prevent the introduction of counterfeit parts into the supply chain.
Risks and Opportunities Applied to Cybersecurity
Evaluation of risks and opportunities is also a function of an Information Security Management System (ISMS). ISO 27001 uses risk assessment to protect information assets. This is accomplished by examining security risks to data that is both digitally and non-digitally stored and then designing and implementing appropriate controls to mitigate these risks.
Applicable controls for data protection include IT solutions, physical site security, and policies. These controls are coordinated by management processes that ensure that these elements continue to provide adequate protection.
Information security is important to an organization’s customer base, supply chain, and partners. Certification in this standard, therefore provides business opportunities and a competitive edge over competition.
Applications for Regulatory Compliance
Organizations are subject to a growing number of regulations and laws, regardless of their business sector. Here again, risks and opportunities play a key role in the establishment of effective policies and procedures. To address these concerns ISO 37301 provides a management tool to coordinate compliance management. Proper application of this standard can help prevent breaches in compliance and provide increased stakeholder confidence.
Improving Business Performance
Regardless of an organization’s industry, having a comprehensive QMS helps your managers to raise the organization’s performance above and beyond competitors who aren’t using management systems. It establishes criteria for expectation for your supply chain in terms of product quality and dependable delivery. It also instills confidence in your brand by consistently meeting customer requirements.
This is because an effectively designed QMS identifies risks and establishes monitoring, measurement, analysis and evaluation to assess the performance of processes. These processes are audited on a regular basis and results from these audits are reviewed by management so that non-conformities can be addressed with corrective actions.
Additionally, the entire QMS system is to be continually reviewed for its effectiveness so that improvements and enhancements can be made when opportunities arise.
CVG Strategy Experts
Our Exemplar Global Lead Auditor Consultants can help you with implementing a quality management system, which will include a risks and opportunities procedure. CVG Strategy has prepared, trained and implemented quality management systems for manufacturing companies in the past 10 years.
CVG Strategy is a consultancy offering coaching, mentoring, training and program development focused on areas including Business Process Improvement, ITAR and Export Compliance, Cyber Security and Product Test and Evaluation.


